Medicines
4 Drivers of Childhood Cancer Treatment Supply Chain Vulnerability ...
Persistent and long-lasting shortages of childhood cancer treatments undermine the advancements in science that have helped to increase childhood cancer survival rates to 85 percent.
How medicine quality affects antimicrobial resistance and the medicines supply chain ...
Supply chain vulnerabilities exist for antimicrobial medicines: USP Medicine Supply M...
Best Practices in State-Level Procurement in India During the COVID-19 Pandemic: Adap...
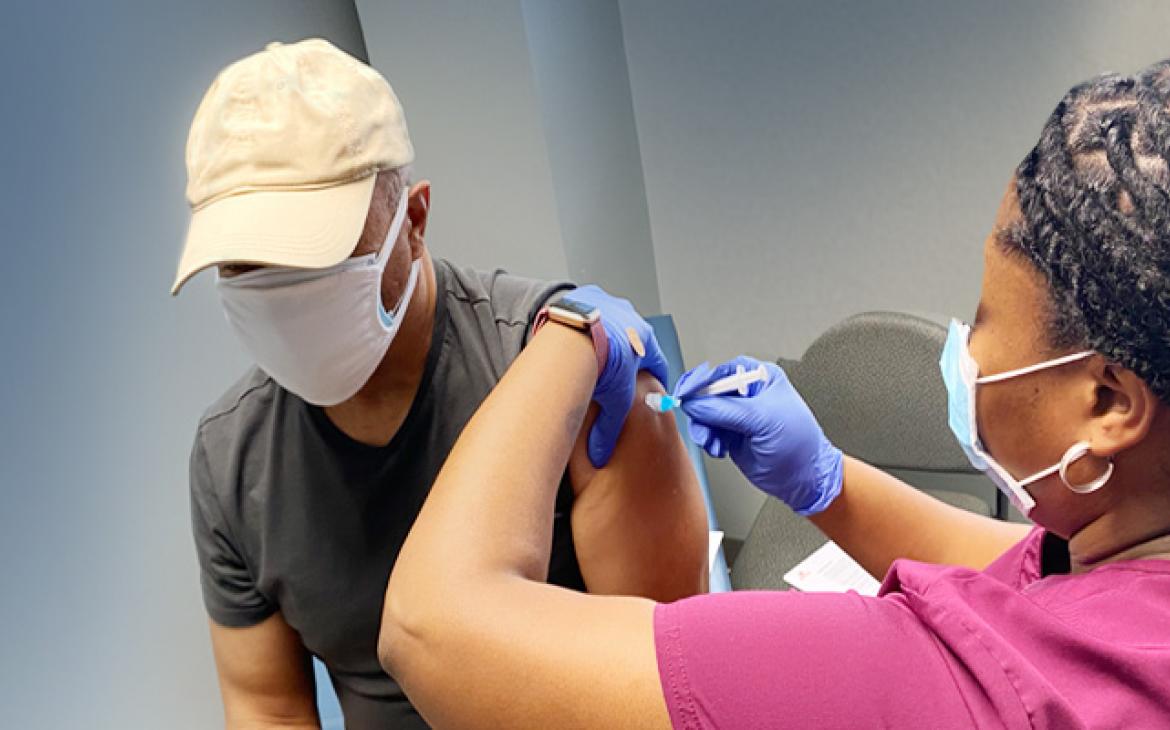
Manufacturing COVID-19 vaccines in Africa
While the global vaccination effort to curb the spread of COVID-19 continues to ramp up worldwide, access to COVID-19 vaccines, particularly in low- and middle-income countries, remains staggeringly low. In fact, low- and middle-income countries received less than one percent of available doses.
USP Volunteer Profile: Developing the COVID-19 Vaccine Handling Toolkit...
USP’s success in its mission to improve global public health through creation of standards and related programs depends in large part on the work of volunteers.
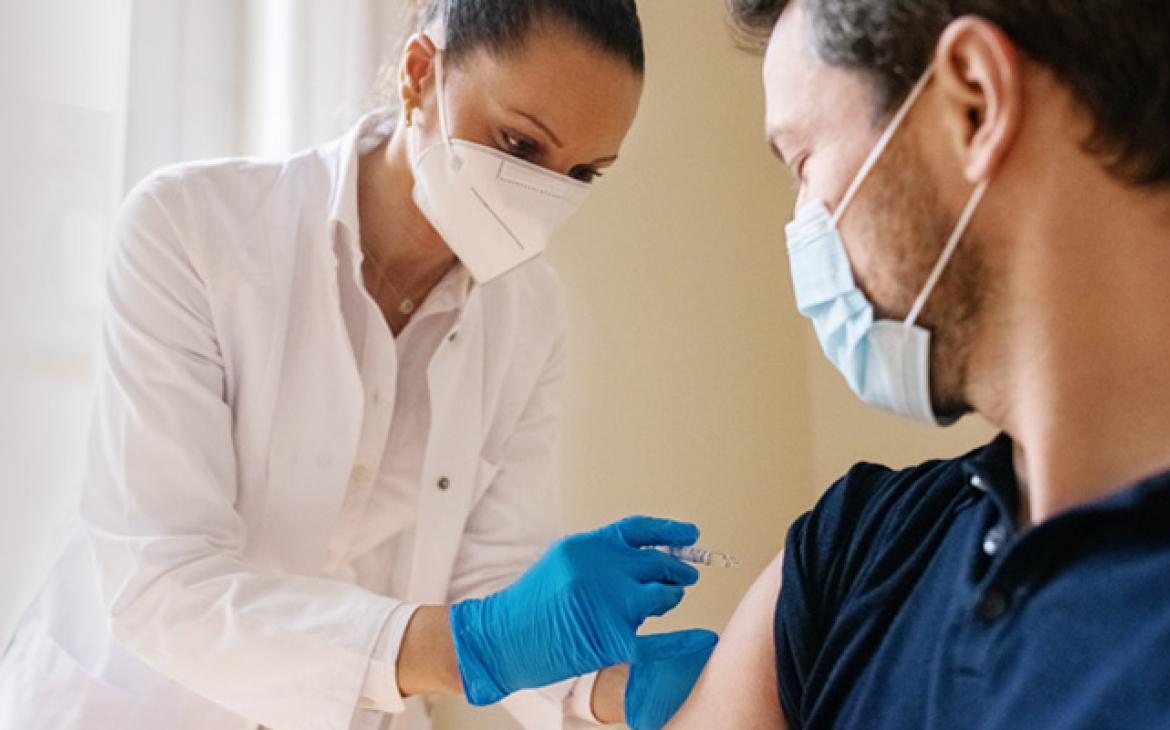
COVID-19 Vaccine Handling: Maximizing Doses & Public Confidence...
USP’s COVID-19 Vaccine Handling Toolkit is designed to help address the challenges nurses and other healthcare providers face every day in preparing, distributing and administering essential vaccines.
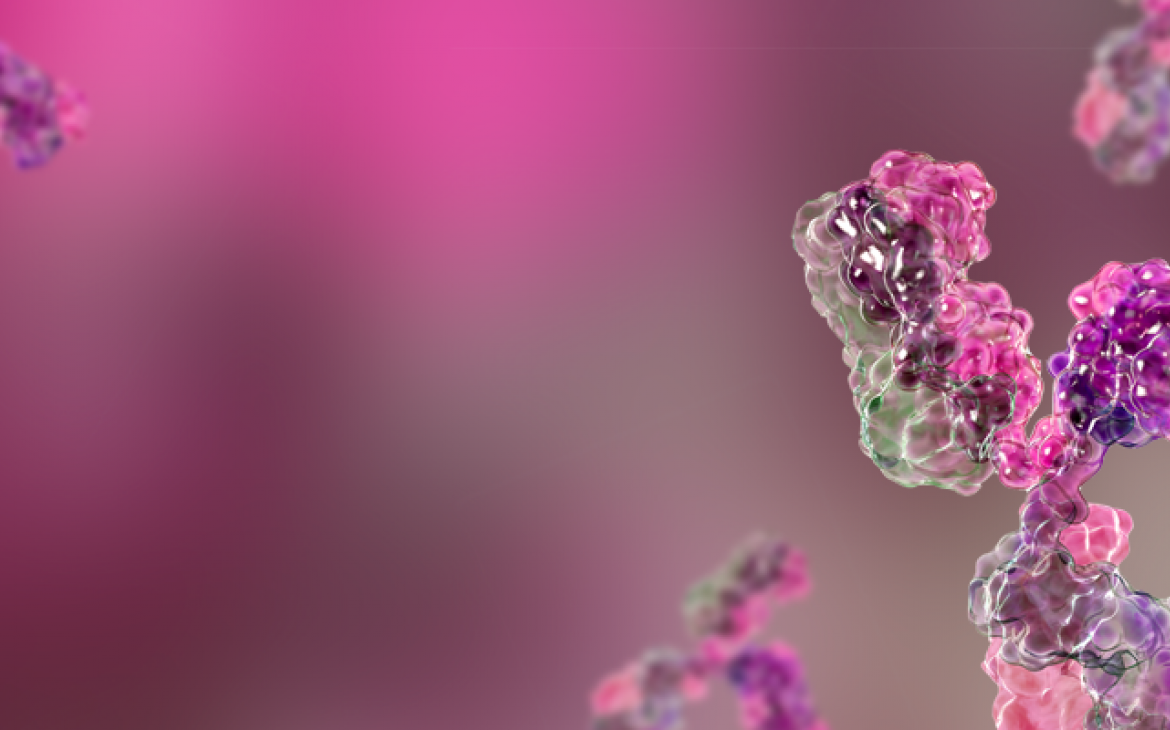
Quality Raw Materials & Collaboration Essential to mAb COVID-19 Treatment Access in L...
Access to quality raw materials and increased stakeholder collaboration are needed to help ensure availability of monoclonal antibody (mAb) treatments for COVID-19 in low- and middle-income countries (LMICs).




 Show 11 More
Show 11 More