The quality of medicine and how it’s delivered to patients is fundamental to treating illness and maintaining health. At USP, we help build a safety net across the drug industry and healthcare system to preserve the well-being of patients. Our established standards help ensure medicines meet quality expectations from the time they are made until the moment they are taken by the patient.
As science continues to evolve, so must the standards USP creates to help healthcare practitioners and organizations stay current to help ensure quality of medicines. USP has long published standards for compounded preparations since the first Pharmacopeia in 1820. USP published its first enforceable chapter on compounding in January 2000, as authorized by the Food and Drug Administration Modernization Act of 1997. The chapter was subsequently revised in 2004 and 2011. USP standards are in a constant state of revision as standards are updated to reflect new science and clinical practice.
When it comes to the development and maintenance of quality standards, we believe public input is critical to ensuring our standards have the intended effects of advancing quality and reducing patient risk. In line with our standard-setting process, when revising the compounding standards, we published the chapter for public comment to obtain input from stakeholders including patients, healthcare practitioners, policymakers, academicians, and industry to ensure a wide variety of perspectives were included in the revised standard.
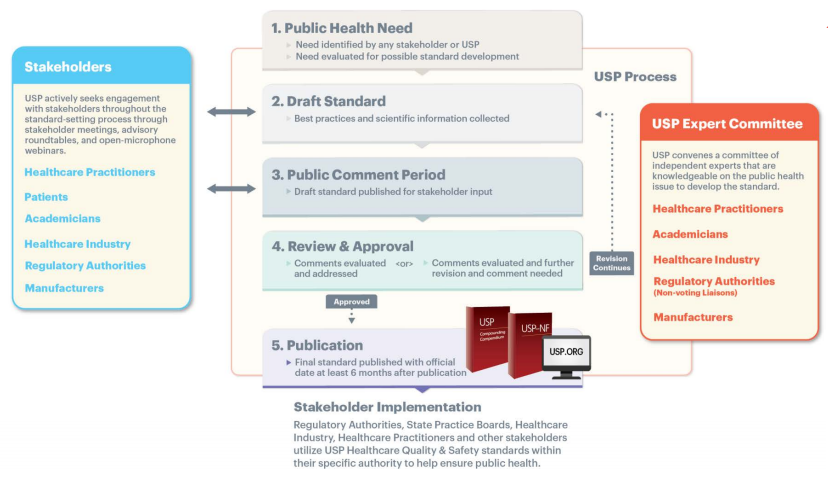
Over the course of a year, our USP Expert Comittees evaluated feedback and discussion from roundtables, discussion forums, and expert consultants. In addition, they reviewed more than 8,000 comments from various stakeholders and diligently worked to engage them in our standards-setting process to help maintain patient access to quality compounded medicines.
I’m proud to report that on June 1, 2019, we published revisions to the standards on compounding nonsterile drugs (<795>) and sterile drugs (<797>), and added a new chapter (<825>) on handling radiopharmaceutical drugs. The updated chapters align with the previously published standards pertaining to the safe handling of hazardous drugs (<800>). The official date for implementation of these four chapters is December 1, 2019.
The implementation of these revised and new compounding standards was truly a community effort. The revisions to the standards:
- Reflect advancements in science and clinical practice;
- Incorporate expert volunteer, stakeholder, and public input to define uniform approaches to help reduce patient risk while maintaining patient access to quality medicines;
- Clarify topics that are frequently misunderstood by healthcare workers; and
- Provide a joint basis from which to help ensure compounded medicines are safe for patients and to help protect healthcare workers if the drugs that are compounded are hazardous.
We appreciate the thoughtful feedback from stakeholders within the public health community. We’ve all done our part to help ensure the quality of compounded medicines for patients with unique needs.
The updated and new compounding standards are available now for FREE digital download.


