
Consumers in the United States often have access to a lot of information about the products they use and consume. Many products include information about the country of origin. Certain brands of cotton apparel even tell you what farms were used to make the cotton. Bedding, furniture and car seats include law labels to inform consumers of filling materials included in the items and the manufacturer. And food labels generally provide ingredients and nutrition facts.
However, despite the large amount of information available about various consumer products, there are still gaps in information for product risk that play a vital rule in public health: medicines.
USP did an analysis of structured product labels approved for use in the U.S. The data below highlights gaps in information publicly available on who manufactured the drug. In 25% of the labels, there was no information available on manufacturers. Only 3% of the labels indicated who had made the Active Pharmaceutical Ingredient (API), a key component in the finished pharmaceutical product.
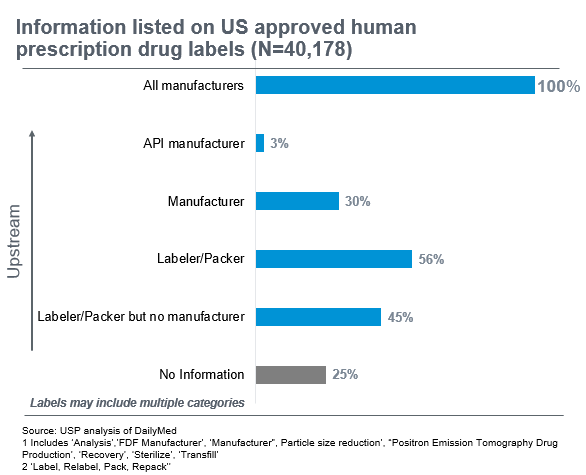
All labels are required to tell you who the “sponsor” of the drug product is, i.e. the company that received approval from the US Food and Drug Administration (FDA) to market the drug in the US. However, in many instances, the manufacturer making the drug product is different than the sponsor because the sponsor may have outsourced the manufacturing. There may also be labelers and repackers involved along the way. In fact, 56% of labels indicated a labeler or packer was involved. The result is a complex and “long” supply chain that is hard to piece together.
Why is this information important for stakeholders to have?
First, there are direct implications for patient safety. A lack of clarity on the supply chain leads to a poor understanding of the risks that may be inherent. As a consequence, providers and patients have practically no advance notice that there may be a problem in the supply chain that could lead to a drug shortage. This can impact patients’ health and likelihood of survival – as we saw when the critical chemotherapy drug used to treat childhood cancer, Vincristine, went into shortage in 2019.
Second, providing this information to manufacturers enables them to take an active role in protecting the resiliency of their own supply chains by identifying high risk drug products that might require additional redundancy built in.
Third, having information about the risk and resilience of the medicine supply chain available to providers, distributors and group purchasing organizations (GPOs) enables them to reward manufacturers that invest in making their supply chains more resilient. In the absence of this information, there is a “race to the bottom” where manufacturers must compete on price alone, rather than supply chain resilience.
What is the solution?
The current vulnerabilities in the supply chain, including the lack of transparency, are the result of numerous factors, requiring multi-faceted solutions. USP has recently published a paper identifying key elements that would improve resilience in the medicine supply chain. The challenge isn’t insurmountable but will require active engagement by all parts of the supply chain, including manufacturers, distributors, policy makers, regulators and public health experts.
USP is supporting improved visibility into potential supply chain risks through the Medicine Supply Map, a data analytics platform that identifies, characterizes and quantifies certain risks and resilience in the upstream supply chain. The Medicine Supply Map arms regulators, providers and other stakeholders with information about certain risks in their supply chain so they can take mitigative action to protect patient access to quality medicines.


