I was pleased to address the American Association of Pharmaceutical Scientists (AAPS) on May 12 during a webinar focused on the impact of the COVID-19 pandemic on the supply of quality medicines. The complex global medicine supply chain is of great interest to the AAPS membership, which includes thousands in academia, science, government, and more.
Since our founding in 1820, USP’s mission has centered on improving global health through public standards and related programs that help ensure the quality, safety, and benefit of medicines and foods. As part of our mission, we work closely with regulators, manufacturers, practitioners, and other stakeholders to establish standards, advocate, and build stakeholder capabilities to help ensure the supply of quality medicines.
USP supports a comprehensive public policy framework to build a more resilient supply chain, as we shared in this recent position paper. We identify 11 key elements needed to accomplish this, including the need to create more geographic diversity, invest in more manufacturing capacity for critical medicines, facilitate more transparency and data sharing worldwide, create crisis contingency plans, and strengthen regulatory systems and quality assurance around the world. USP is actively collaborating with our industry and regulatory partners to strengthen these efforts, particularly as we support new potential therapies for COVID-19.
Using data to create greater transparency
Creating more transparency to identify potential geographic concentrations of critical medicines is a vitally important aspect of improving the resiliency of the supply chain. USP and many other entities have found there is very limited data on where medicines are made. More access to data will help us all better manage risks that could interrupt the supply of quality medicines for all. We began to look at the data we have collected through our own work to start identifying where there are potential vulnerabilities — and strengths — in the global supply chain.
USP provides reference standards, which more than 22,000 entities used in 2019, to help ensure quality in pharmaceutical manufacturing, regulatory quality assurance, and more. Demand for USP reference standards — as well as the on-the-ground intelligence that we have access to through our global network of staff, volunteers, and Convention members — allow us to see supply chains with more clarity than most organizations can.
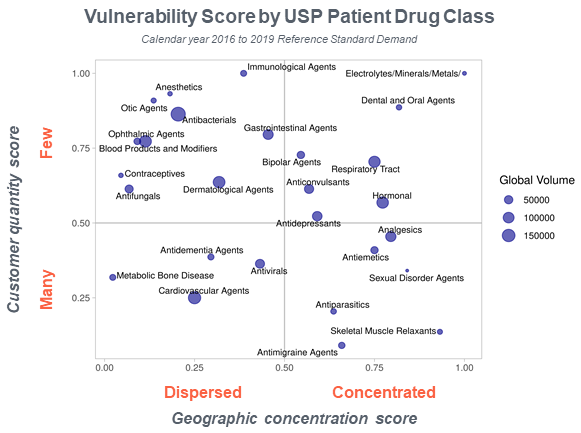
For example, an analysis of demand for our reference standards data shows that while there are many manufacturers of cardiovascular agents worldwide, the same is not true for anesthetics. We can also see that anesthetic agents are made in very few countries, which renders them more vulnerable to supply chain disruptions. There are several other factors that make the supply of medicines vulnerable, including market size (smaller markets are less attractive to manufacturers) and manufacturing complexity (drugs that are more difficult or expensive to manufacture are more vulnerable to supply chain interruptions).
Moving forward, USP will expand on the current analysis of our reference standards data and link it to data from a variety of other sources. We’re partnering with sophisticated data scientists to do this, and we look forward to sharing what we learn in the coming months. While maintaining sensitivity to any proprietary data, we plan to develop actionable insights that can be used to improve transparency on a global scale and benefit manufacturers, regulators, practitioners — and, ultimately, patients. We plan to share what we learn as we go, so we can get feedback to help improve the information we provide.
Diversifying manufacturing methods to improve supply chain flexibility
In addition to transparency of data, we believe USP’s ability to facilitate faster adoption of new manufacturing technologies will strengthen the resilience of the supply chain. As one example, USP’s inclusion of high-performance liquid chromatography (HPLC) methods in our standards is tied to an increased use of HPLC in literature. This demonstrates that USP standards can encourage the adoption of new technology by industry to help ensure quality medicines.
At AAPS, I was joined by Frank Gupton, Ph.D., Founder and CEO of the Medicines for All Institute. He shared some of the groundbreaking work they are doing to increase access to essential medicines, largely through faster, more efficient, and more cost-effective pharmaceutical processing. They are also working on novel approaches to chemistry and looking at ways to make the most of the institute’s global network of chemical expertise to transform pharmaceutical process development by leveraging new manufacturing technologies.
Dr. Gupton and I talked about upstream supply chain transparency, which he sees as having two very clear goals: building manufacturing capacity for critical medicines and investing in advanced manufacturing technologies. This includes pharmaceutical continuous manufacturing, which has several advantages over batch manufacturing. Continuous manufacturing gives manufacturers the flexibility to produce different quantities of product with more consistent quality and significantly reduces the manufacturing footprint as a way to lower costs.
Dr. Gupton believes the impact of Medicines for All could bring both a short- and a long-term solution to supply chain disruptions. In the short term, developing more robust chemistry that can be quickly implemented in available manufacturing facilities could be the way to go. Ultimately, greater automation and flexibility — which advanced manufacturing approaches, such as continuous manufacturing, can make possible — will strengthen manufacturing capacity and capability. Continuous manufacturing units have a significantly smaller physical footprint, so they can be easily replicated elsewhere as a way to address local drug shortages. Given that more than 90% of prescription drugs dispensed in the U.S. today are generics, the adoption of continuous manufacturing will greatly improve the availability of quality, affordable medicines essential for patients. USP is committed to providing resources to facilitate that.
This is just the start of the process. We believe that even early data can be as valuable as a substantial mapping of vulnerability and strengths. USP stands ready to partner with stakeholders on solutions for a transparent and resilient supply chain. We also support a comprehensive public policy framework to build a more resilient supply chain, including advancing the use of pharmacopeial standards, to help ensure the supply of quality medicines around the world.


