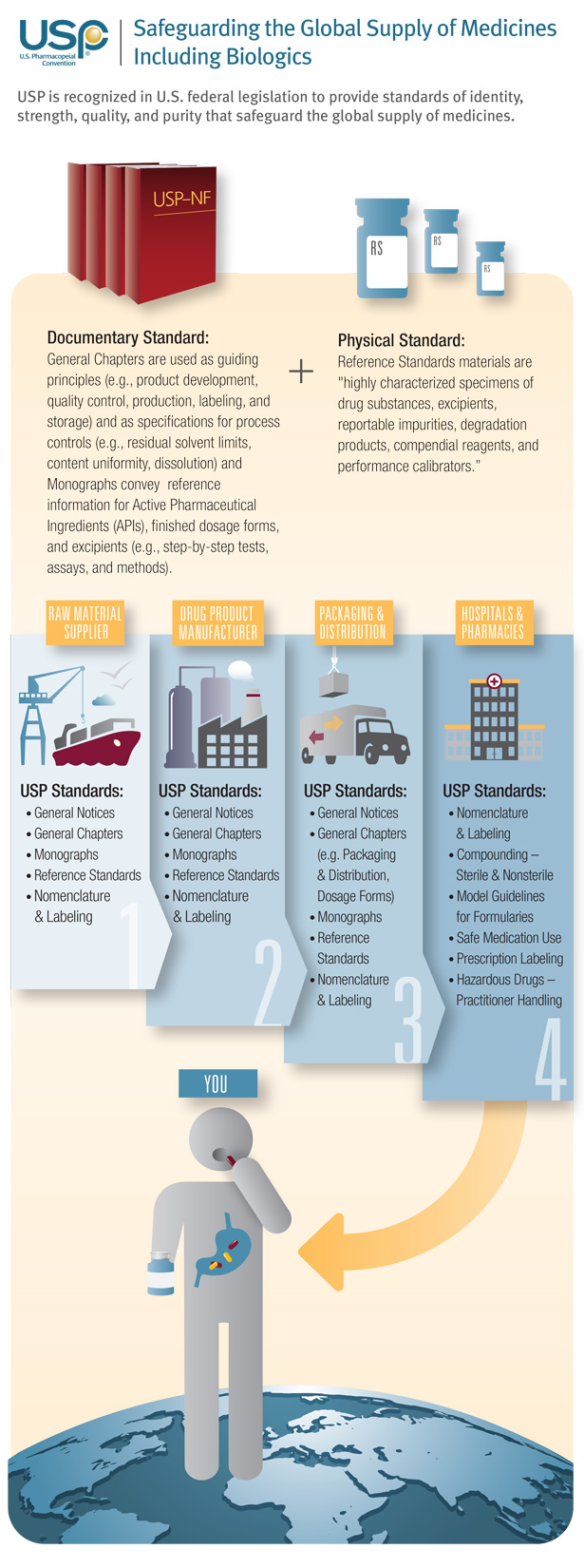
Today, many critical, life-saving medicines go through a complex global supply chain before ever reaching the patient. USP’s public standards play important roles throughout that supply chain. These range from standards that ensure the quality of raw materials used to make ingredients that go into drugs to healthcare quality practice standards that ensure that pharmacists, physicians and nurses are delivering high-quality care to patients.
In the field of biologic drugs—medicines derived from living organisms—USP standards are relevant throughout the entire life cycle of a therapeutic product. USP’s standards include measures for analyzing the quantity and type of unwanted microbial content; guidelines on good packaging, storage and distribution practices; dosing requirements to help avoid medication errors and other quality-related expectations on proteins, DNA residue, bioassays and other parameters that impact biologic drug quality.
Learn more about the role of USP standards in the manufacture, product surveillance and administration of biologic medicines.


