
When it comes to good distribution practices (GDPs) for medical products, patient safety is the highest priority, panelists affirmed during an April 26 session of the Asia-Pacific Economic Cooperation (APEC) forum’s Medical Product Supply Chain Dialogue, co-hosted by the U.S. FDA and USP in Rockville, Maryland. To ensure GDPs and patient safety requires coordination, collaboration, and continuous quality improvement, the panel generally agreed. The panel was titled: “Securing the Downstream Supply Chain: Good Distribution Practices.”
From the moment a pharmaceutical product leaves a manufacturing plant until its delivery to a patient, GDPs play a vital role in a secure medicines supply chain. They help to ensure drug quality is preserved during transportation and storage, for example, by addressing risks that can stem from potential exposure to extreme temperatures and humidity, tampering, or unauthorized diversion. During the panel discussion, participants underscored the need for GDPs to safeguard drug quality through the last mile of the supply chain to support patient and healthcare provider trust in quality-assured medicines.
GDPs address supply chain risks
The challenge of maintaining GDPs is magnified by the inherent complexity of today’s global drug product supply chain, with multiple stakeholder touchpoints including manufacturers, distributors, third-party logistics suppliers, pharmacies, hospitals, and clinics, as shown in Figure 1 below.
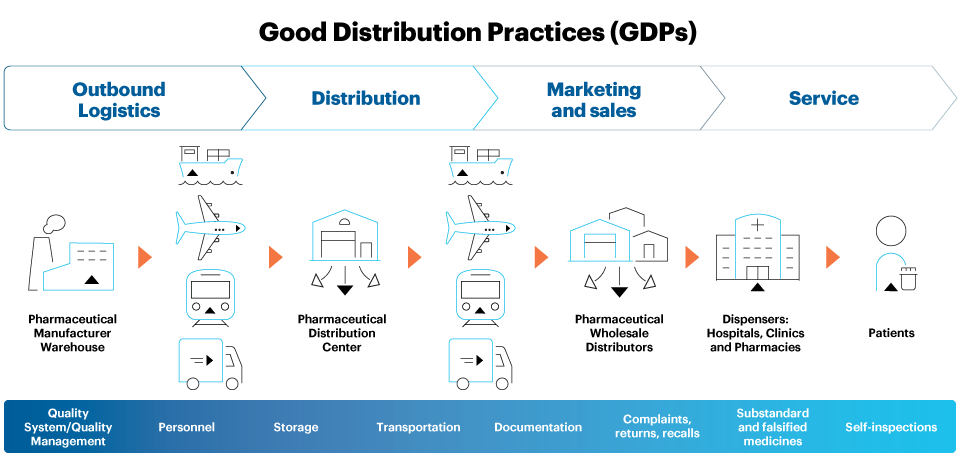
Figure 1. An illustration of key medicines supply chain touch points, processes, and risks requiring GDPs
With multiple exchange and drop-off points, potentially including several modes of transportation, climate zones, and seasonal changes – as well as other environmental factors such as light exposure, shock, vibration, and compression – finished medicines can face a gauntlet of risk that makes GDPs essential to maintaining product quality. Beyond addressing proper documentation, storage, and transportation, GDPs incorporate appropriate mitigation strategies to control the foreseeable risks.
USP provides standards to help ensure quality across the pharmaceutical product lifecycle – including distribution – from the sourcing of raw materials to production and point-of-care administration. Such standards help reduce health disparities, ensure health equity, and bolster supply chain resilience around the world.
“The more complex the distribution model…the more likely that you are going to have a failure along the line,” asserted panelist Jennifer Stone, senior vice president of quality at PTC Therapeutics, a developer of drugs for rare diseases. Spotlighting one example of distribution model complexity, Stone recounted the case of a drug intended to be shipped from one country to another that in fact was routed to multiple different countries around the world before eventually being delivered to a patient. Such cases demonstrate the need for vigilance and ongoing stakeholder efforts to ensure GDPs and timely access to quality-assured medicines.
Coordination stems counterfeiting
With so many stakeholders involved in distribution, efforts to keep potentially substandard and falsified medicines from entering the medicines supply chain requires coordination and collaboration both internally within companies and externally with third parties including governments and regulatory bodies. Panelists particularly emphasized the value of collaboration among stakeholders including USP, the U.S. Food and Drug Administration (FDA), and global regulators.
According to the Pharmaceutical Security Institute, incidents of counterfeiting, illegal diversion and theft of medicines totaled over 5,900 in 2021. Out of the 550+ reported counterfeiting incidents, either customs seizures or police/health inspector raids were involved, and 60% of the seizures comprised more than 1,000 dosage units. This amounts to more than 300,000 doses of counterfeit drugs intercepted. Such quality issues contribute to the risk of drug shortages that also can impact patient health. Pre-pandemic, quality problems were responsible for the vast majority of drug shortages – at over 60% – according to a 2019 FDA analysis.
During the APEC meeting, panelist Suzana Yumi Fujimoto, deputy director of the Brazilian Health Regulatory Agency (ANVISA) outlined progress Brazil has made in advancing GDPs since the late 1990s. This has included a focus on storage and transportation practices for medicinal products related to quality management, training, capacity building, monitoring, continuous improvement, and enforcement. Through implementation of GDP regulations, supply chain security methodologies, and a focus on patient safety, ANVISA has helped protect and promote public health in Brazil, Fujimoto said.
Standards ensure quality
The USP presentation to the panel included an overview of USP's GDP-related milestones and current approach, which focuses on risks and mitigation strategies for the storage and transportation of drug products. The presentation outlined the proposed revision of USP General Chapter <1079.2> Mean Kinetic Temperature in the Evaluation of Temperature Excursions during Storage and Transportation of Drug Products. The revision would include temperature excursion limits for climatic zone IVb to address a current gap, as it currently contains temperature excursion limits for climatic zone II. The review was identified as a necessity, considering collaborative work with several Brazilian stakeholders – including ANVISA, manufacturers, distributors, and transporters – and Brazil’s location in climatic zone IVb. An expanded rationale for the revision was included in a related Stimuli article.
When in-country supply chain partners lack access to manufacturer stability studies, the mean kinetic temperature associated with temperature excursion limits can be a tool to assess the severity of the excursion and the need to implement thermal solutions.
APEC economies also located in climate zone IVb could benefit from the revision of USP <1079.2>. The APEC Supply Chain Security Toolkit underscores that GDPs should be followed by all stakeholders as medical products move through the supply chain to help bolster patient safety.
Continuous improvement is essential
The importance of building a culture of continuous improvement throughout the supply chain was another theme that emerged during the panel. GDPs implemented through the quality management system of each medical product company ensure companies meet minimum standards while continuously improving their practices for better efficiency. The rapid development of sophisticated medical products with specific environmental needs presents new challenges, making it crucial to prioritize continual improvement and adapt to emerging commercial and legislative demands for achieving success. Identifying adverse events, looking at signals and trends, investigating irregularities, and evaluating how to enhance surveillance as products move through the distribution chain can help companies, industry partners, and regulators support product quality and supply chain resilience.
Lessons learned aid preparedness
During the COVID-19 pandemic, many distributors were able to quickly pivot to meet unique vaccine storage and temperature requirements. As regions around the world emerge from the public health emergency, leveraging the lessons learned will be key to bolstering preparedness for future pandemics. In its efforts to meet evolving stakeholder needs related to GDPs, USP is working to revise related standards, including USP <1079.2>. In addition, USP has developed new General Chapter <1079.3> Monitoring Devices – Time, Temperature, and Humidity. Also open for public comments is new General Chapter <1079.4> Qualification of Storage Areas. Other general chapters are also in development that cover topics such as the qualification of packaging systems for distribution, lane mapping, and information systems for distribution validation/verification studies.
Going forward, USP will continue to evaluate and address opportunities to meet evolving stakeholder needs and emerging public health priorities through standards and other quality-focused solutions.


