
Understanding the landscape to expand, strengthen, and diversify African-based manufacturing
Every two minutes, a woman dies during pregnancy and childbirth, according to the World Health Organization (WHO). Yet, most of these deaths could be prevented with better access to simple, widely available maternal health (MH) products. While challenges to access have long existed in sub-Saharan Africa (SSA), supply chain issues for pharmaceutical products were greatly exacerbated during the COVID-19 pandemic.
Disruptions in supply chains, reliance on foreign imports, and limited local production impede availability of lifesaving maternal health products for women across sub-Saharan Africa.
Following COVID-19, countries across Africa found that health supply chains were not sustainable or capable of meeting the needs of their people, especially in times of crisis. This led to new commitments and action to expand Africa’s industrial capacity for pharmaceutical manufacturing.
Five products, eight countries
Broadly speaking, USP and RHSC seek to promote healthier markets, including through growth in regional manufacturing of health supplies in SSA. To that end, USP and RHSC released a report, Manufacturing landscape assessment for maternal health supplies in sub-Saharan Africa, which provides a path forward for creating more stable and resilient supply chains for MH products.
In this report, we analyzed the supply and demand of five high-priority MH commodities, which include: heat-stable carbetocin (HSC), magnesium sulfate, misoprostol, oxytocin, and tranexamic acid (TXA). The geographic scope of the analysis was eight countries in SSA: Ethiopia, Ghana, Kenya, Nigeria, South Africa, Tanzania, Uganda, and Zimbabwe.
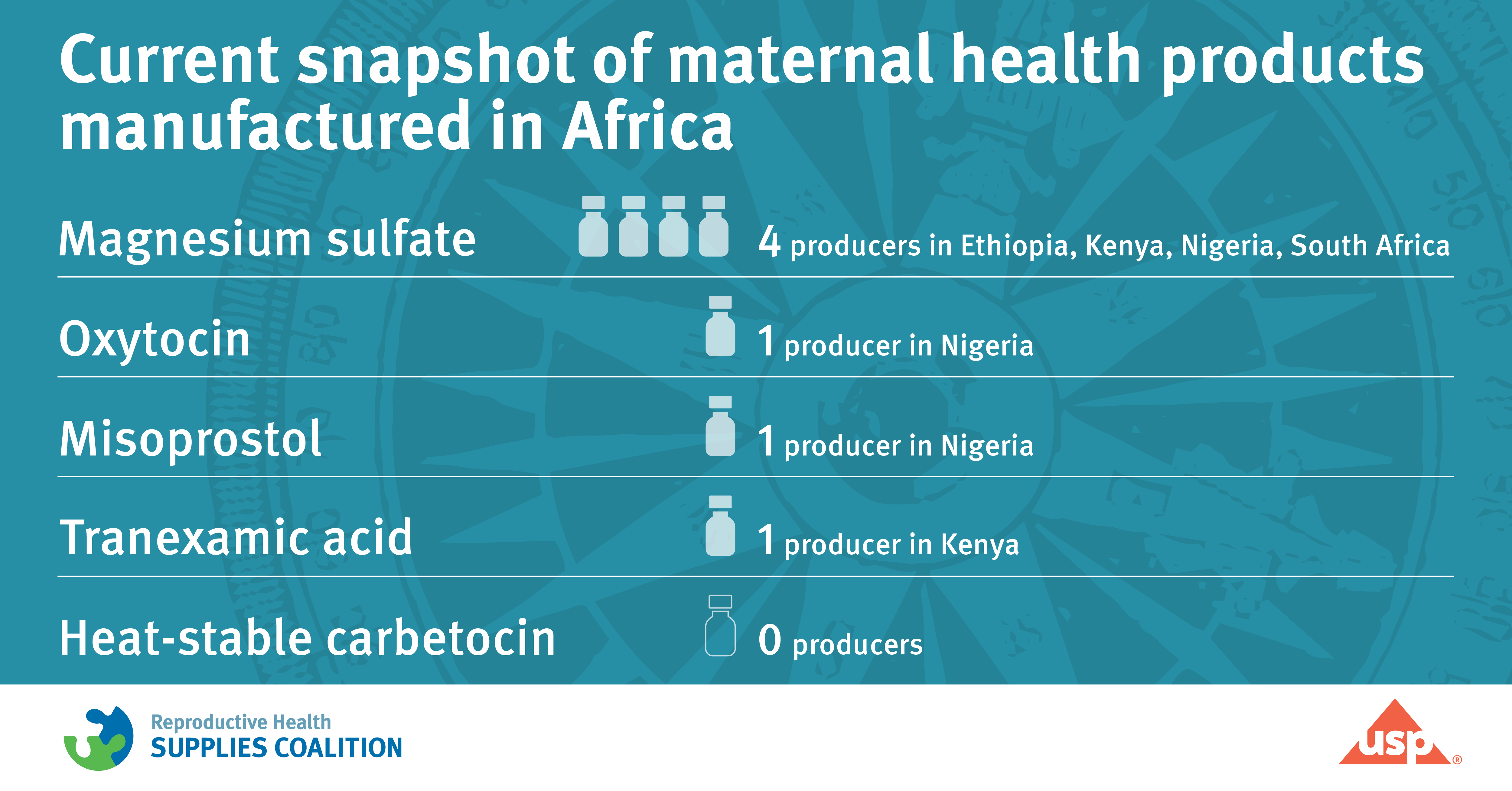
More diverse, African-based producers needed
Overall, we found six manufacturers from four countries – Ethiopia, Kenya, Nigeria, and South Africa – actively involved in the production of MH products. We also found that four of the five MH products analyzed were either produced by a single manufacturer based in SSA or none at all. Only one product was manufactured by multiple African-based producers; however, those manufacturers are currently experiencing significant challenges with production capacity and distribution.
For example, only one Nigerian manufacturer, Juhel, produces oxytocin. Oxytocin is an inexpensive, widely available, and effective medicine to prevent and treat PPH, which faces cold storage challenges within the supply chain. In addition, one producer in Kenya, Tasa Pharma, manufactures tranexamic acid (TXA), which also helps treat PPH.
Misoprostol, which can be used to prevent and treat PPH in hospital and community settings, is only manufactured by one regional producer, Emzor, based in Nigeria.
All three African-based manufacturers of oxytocin, TXA, and misoprostol face logistical challenges in scaling up production. With so few African-based manufacturers producing these essential MH commodities, the region relies heavily on foreign imported brands, including from India and China, making SSA more vulnerable to potential disruptions in the supply chain, like those we saw during the pandemic.

Expanding production of MH products
In this study, we explored the capacity of existing regional manufacturers of MH products, as well as the potential for other regional manufacturers, to add one or more of these MH products to their portfolios. Of the African-based manufacturers identified in the study, nearly all have relatively low factory utilization rates, which means they have room to expand their portfolio of MH products. This includes three manufacturers that hope to produce heat-stable carbetocin (HSC), which helps prevent PPH, but unlike oxytocin, does not require refrigeration.
HSC is not yet manufactured in SSA. And there is opportunity for countries to add the product to more essential medicines lists and national registries.
Gaps and challenges
Magnesium sulfate, which aids in treating eclampsia or seizures due to high blood pressure that occur during pregnancy or after birth, is produced by four manufacturers, Humanwell Pharma, Laboratory and Allied Health, Juhel, and Adcock Ingram, in four countries in SSA – Ethiopia, Kenya, Nigeria, and South Africa respectively.
However, despite the presence of these four regional manufacturers, we found that magnesium sulfate is still at risk of supply chain disruptions, in part due to limited production capacity and a need for greater product distribution.

All four manufacturers formulate and package finished pharmaceutical products (FPPs); none are limited to only packaging services, a sign of growing maturity. However, none of the manufacturers currently supply their products to neighboring countries or regions in Africa. Common challenges hindering distribution across borders include the unpredictable nature of regulatory frameworks, registration delays, inadequate transportation infrastructure, and the lack of a regional procurement mechanism.
Next steps
The report provides an in-depth understanding of the MH product landscape in SSA, highlighting potential vulnerabilities and opportunities to strengthen regional manufacturing and ensure a more resilient and diversified supply chain.
Moving forward, manufacturers across Africa are committed to increasing the production of essential MH commodities and addressing critical gaps in supply. To that end, we recommend continued collaboration between local governments, regional bodies, and international organizations to overcome challenges and foster a more robust MH product manufacturing industry.
In addition, strengthening business and regulatory environments and improving access to regional and continental markets will be crucial to ensuring the availability and affordability of these lifesaving MH products across the region.
This post also appears on It's about Supplies, a blog from the the Reproductive Health Supplies Coalition (RHSC).




