From the Field: USP Vaccine Handling Toolkit Is Shot In The Arm For Administration E...
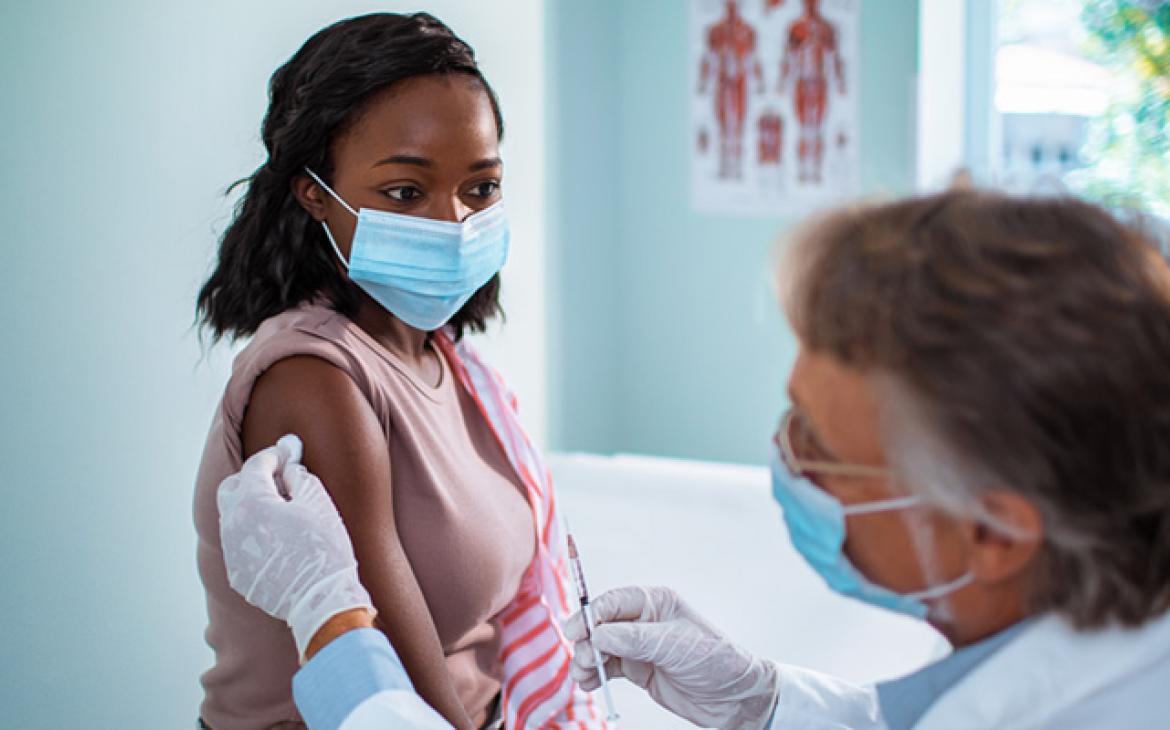
USP’s recently released COVID-19 Vaccine Handling Toolkit is helping to fill critical operational efficiency gaps that practice settings have identified in vaccine administration.





 Show 11 More
Show 11 More